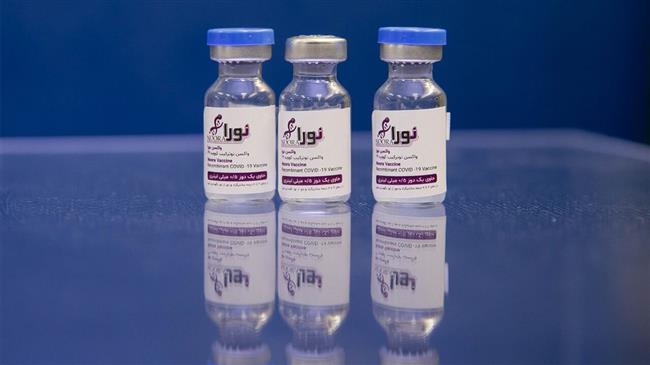
Iran’s home-grown Noora COVID-19 vaccine enters second stage of human trial
A senior official with the Islamic Revolution Guards Corps (IRGC) says Iran’s domestically-developed and produced Noora coronavirus vaccine has entered the second stage of human trial, only a day after Iranian scientists began the third phase of the clinical trial of homegrown Razi Cov Pars COVID-19 vaccine.
Brigadier General Ahmad Abdollahi, the IRGC’s Deputy Director of Health, Medical Education and Biological Defense Affairs, said on Monday that the second phase of the clinical trial of the recombinant Noora vaccine has started.
“We hope to make this vaccine available to the public once the second and third stages [of human trial] have been carried out,” he noted.
Noora vaccine, produced by Baqiyatallah University of Medical Sciences, was put on display during a ceremony in Tehran on June 27 in the presence of Chief Commander of the IRGC Major General Hossein Salami, Iran’s former health minister Saeed Namaki as well as other Iranian health officials.
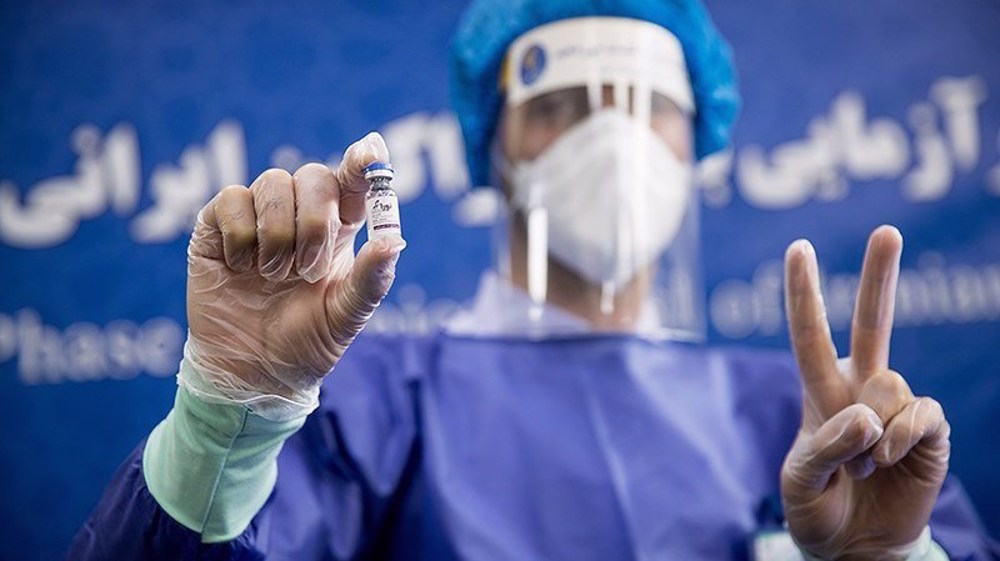
The recombinant vaccine entered the first stage of human trial after 16 months of research work by Iranian scientists.
Its first dose was injected into the chief medial officer of Baqiyatallah Hospital, Dr. Hossein Samadinia.
“It is noteworthy that a few universities in the world have been able to develop a vaccine against the virus, and it is a great honor for the Baqiyatallah University of Medical Sciences to be able to achieve this success,” Samadinia said on the sidelines of the unveiling ceremony at the time.
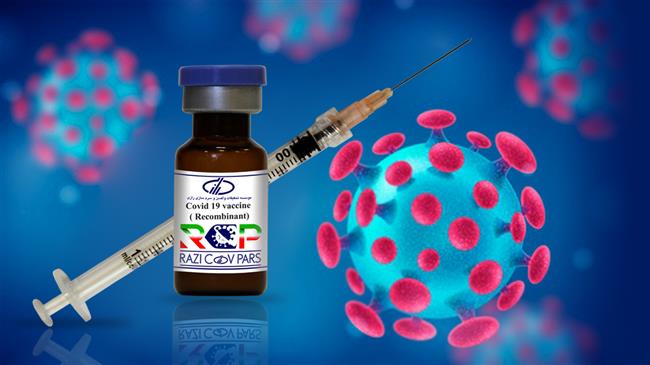
On Sunday, Iran started the third phase of the human trial of its second homegrown coronavirus vaccine, Razi Cov Pars.
Razi Cov Pars is a recombinant protein subunit vaccine containing the COVID-19 spike protein. It reportedly tutors the immune system against the virus by producing antibodies.
The vaccine includes three doses. The first two doses are said to be injectable, whilst the third dose is intranasal.
The second dose of the vaccine will be injected into volunteers 21 days after the first inoculation, and the third dose will be inhaled 51 days later.
Iran has also successfully completed the first phase of the human trial for FAKHRAVAC COVID-19 vaccine developed by the Organization of Defensive Innovation and Research.
FAKHRAVAC is an inactivated virus-based vaccine, and apparently requires two doses given by intramuscular injection 14 days apart.
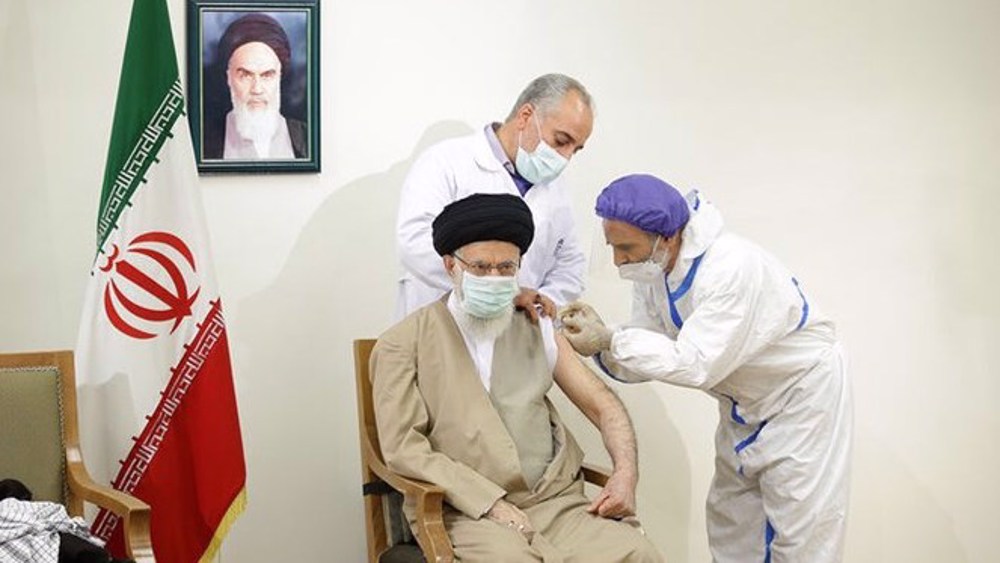
On June 25, Leader of the Islamic Revolution Ayatollah Seyyed Ali Khamenei received his first dose of the CovIran Barekat vaccine, which was approved for emergency mass application by the country’s Health Ministry earlier that month.
After receiving the vaccine, the Leader thanked all those involved in the development of the homegrown vaccine, produced as part of a project led by the Headquarters for the Execution of Imam Khomeini's Order, a charitable organization.
Ayatollah Khamenei thanked the head of the organization and his colleagues as well as Namaki and all of the country’s health workers who have been fighting against the deadly COVID-19 virus for more than a year.
Iran’s political legitimacy comes from ballots and popular will, not foreign powers
VIDEO | Press TV's news headlines
VIDEO | The reckless US
Qalibaf: Recent unrest a 'complementary link' in 12-day war against Iran
Ex-Israeli war minister: Iranian missiles inflicted heavy losses
Trump launches 'Board of Peace' seen as bid to control Gaza
Senior general vows swift response to any aggression on Iran
US federal immigration agents detain 5-year-old boy in Minnesota












 This makes it easy to access the Press TV website
This makes it easy to access the Press TV website